Overture Orthopaedics Wins Octane's 2025 High Tech Award for "Best Innovation in Medical Device"
IRVINE, Calif., Oct. 1, 2025 /PRNewswire/ -- Overture Orthopaedics, a privately held U.S.-based medical device company providing surgeons with innovative joint preservation solutions in sports medicine and orthopaedic surgery, announced it has received the 2025 High Tech Award for "Best Innovation in Medical Device" from Octane, Southern California's premier organization dedicated to driving innovation in the technology and life science industries.
Each year, the High Tech Award celebrates and recognizes a new standout medical technology that will make a significant impact in the healthcare space and raise the standard of care for patients and surgeons. In a group of very impressive finalists, Overture is extremely honored to have won the award in the medical device category.
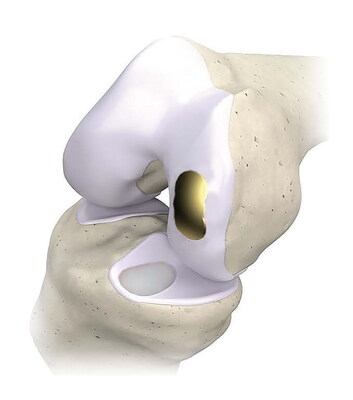
OvertureTi Knee Resurfacing System® implants are designed specifically as an alternative when it is still too early for arthroplasty, but biological repair options are not viable for treating early osteoarthritis and focal cartilage lesions. The system is composed of femoral and tibial implants intended to be used in the partial replacement of the articular surfaces of the knee. These implants were designed with sizing options that allow the surgeon to replace only the diseased or damaged region of the joint while preserving healthy native cartilage and soft tissue. The procedure is referred to as Focalplasty®.
With an ever-increasing active patient population, the number of cartilage restoration surgeries performed annually exceeds 300,000, notably growing each year. A 2021 study on chondral lesions of the knee in the Journal of Bone and Joint Surgery by Dekker et al., highlights current surgical and biological options for treatment of isolated cartilage defects continue to present significant challenges for patients and surgeons in terms of cost and complexity, including inconsistent healing, donor site morbidity, and degradation over time. According to a study by McCormick et al. that focused on trends in the surgical treatment of articular cartilage lesions in the United States, some procedures such as chondrocyte implantation reach costs as high as $83,073 due to multi-stage surgeries, laboratory processing, associated episode of care, and societal considerations.
"Joint preservation is what we've been looking for since day one," said Jorge Chahla, MD, Associate Professor of Orthopedic Surgery at Rush Medical Center in Chicago, IL and Team Physician of the Chicago Bulls and Chicago White Sox. "Overture has a very minimally invasive approach; you don't have to take out any unnecessary cartilage and bone, which is a great thing because you are preserving most of the cartilage and preserving most of the bone in the event the patient needs a replacement in the future. The other important thing is you are not touching any of the ligaments, which we know play a very important role in proprioception, making people feel more normal in their knee. You can achieve normal native biomechanics with Overture, which can be a game changer in joint preservation."
Overture's technologically advanced, biocompatible implants have baseplates that are 3D printed, porous titanium to promote bony in-growth and solid fixation; articulating surfaces for the femoral and tibial components are clinically proven titanium nitride and over-molded highly cross-linked Vitamin E treated polyethylene, respectively. The implants are a fraction of the cost of biological options.
"Being recognized by Octane and the greater community is a rewarding moment for us here at Overture," shared James Young Kim, Overture's Chief Executive Officer. "This recognition reflects the innovation behind our mission to raise the standard of care in joint preservation. We are confident that we are taking the small steps necessary to rethink treatment of the knee, where less can be more. Our technology is helping surgeons make smarter decisions with a new approach to better address knee pain and extend active lifestyles, ultimately raising the standard of care for today's changing patient population."
The OvertureTi Knee Resurfacing System is 510(k) cleared by the United States Food and Drug Administration and available for immediate use, with all instrumentation sterile-packed for streamlined, single-use efficiency in the operating room.
For more information, please email info@overtureortho.com or call 949-889-3784.
About Overture Orthopaedics
Overture Orthopaedics is a privately held medical device company providing sports medicine and orthopaedic surgeons with specially designed implants to relieve pain and dysfunction associated with articular cartilage lesions, osteochondral defects, and early osteoarthritis. We believe that our solutions raise the standard of care in joint preservation by providing state of the art surgical tools that streamline use in the operating room, reduce the global cost of care, and enhance outcomes so that patients can get back to and maintain their active lifestyles longer. For more information regarding Overture Orthopaedics, please visit www.overtureortho.com.
"Overture Orthopaedics®, OvertureTi Knee Resurfacing System®, and Focalplasty® are trademarks of Overture Orthopaedics"
Contact: James Young Kim
Overture Orthopaedics
Tel: +1.949.889.3784
jkim@overtureortho.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/overture-orthopaedics-wins-octanes-2025-high-tech-award-for-best-innovation-in-medical-device-302572824.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/overture-orthopaedics-wins-octanes-2025-high-tech-award-for-best-innovation-in-medical-device-302572824.html
SOURCE Overture Orthopaedics