CAPS Medical's PlasmaSure™ Receives FDA Breakthrough Device Designation
Breakthrough designation for Treatment of Non-Muscle Invasive Bladder Cancer (NMIBC) supports CAPS Medical's mission to transform tumor ablation into a simple, selective, and in-office procedure
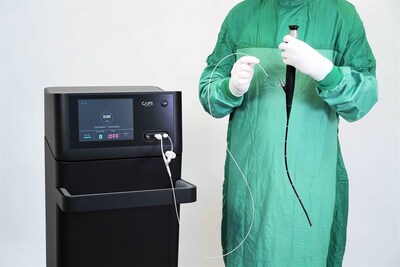
SANTA MONICA, Calif. and NETANYA, Israel, Oct. 15, 2025 /PRNewswire/ -- CAPS Medical, a clinical-stage MedTech company developing a platform for selective tumor ablation across multiple indications, today announced that its PlasmaSure™ System has received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the treatment of low- to intermediate-risk non-muscle invasive bladder cancer (NMIBC). The PlasmaSure™ platform delivers non-thermal atmospheric plasma energy through the working channels of standard minimally invasive tools, in the case of NMIBC, through cystoscopes, enabling highly selective, tissue-preserving tumor ablation.
Transforming NMIBC Care with a Simple In-Office Procedure
CAPS Medical has successfully completed its first-in-human clinical study, treating approximately 70 tumors and demonstrating durable complete response rates across short and long term follow up. The study showed an improved safety profile with no adverse events, no damage to surrounding healthy tissue, and excellent patient tolerance including procedures performed without general anesthesia. The company's goal is to replace traditional transurethral resection of bladder tumors (TURBT), hospitalization, and general anesthesia with a simple, office-based procedure that allows patients to be treated earlier, with less risk, faster recovery, and lower cost.
Ilan Uchitel, CEO & Co-Founder of CAPS Medical, commented: "Receiving Breakthrough Device Designation is a strong validation of the clinical need for a safer, more accessible solution for NMIBC patients and of the differentiated potential of our PlasmaSure™ platform. This designation provides priority review and interactive communication with the FDA, more flexible clinical study design, and promising potential eligibility for CMS's Transitional Coverage for Emerging Technologies pathway, supporting earlier and more predictable reimbursement.Our team is committed to turning this innovation into a new standard of care."
This milestone marks the beginning of the next growth phase for CAPS Medical as we prepare for pivotal trials, U.S. market entry, and global expansion.
Expanding to Multiple Solid Tumor Indications
Building on its clinical success in NMIBC, CAPS Medical is advancing pre-clinical programs in lung, esophageal, gastric, and prostate cancer, among others. Early data from these studies show promising tumor ablation results, reinforcing the potential for the platform to become a multi-indication oncology solution.
Strategic U.S. Presence and Next Growth Phase
To support its next stage of development, CAPS Medical is establishing a U.S. headquarters and preparing for its pivotal study and subsequent commercialization efforts, enabling closer engagement with clinical partners, regulators, and payors in the U.S. market.
About CAPS Medical
CAPS Medical www.capsmedical.com is a clinical-stage MedTech company commercializing PlasmaSure™, a minimally invasive, non-thermal plasma system designed to ablate solid tumors while preserving healthy tissue. CAPS Medical is expanding its technology to multiple high-burden cancer indications, with the mission to make tumor ablation safer, simpler, and more accessible worldwide.
For more information, visit www.capsmedical.com
Follow CAPS Medical on LinkedIn
Contact:
Ido Atiya
ido@idoatiya.com
+972-544-289-205
Photo - https://mma.prnewswire.com/media/2784221/CAPS_Medical_PlasmaSure.jpg
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/caps-medicals-plasmasure-receives-fda-breakthrough-device-designation-302584645.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/caps-medicals-plasmasure-receives-fda-breakthrough-device-designation-302584645.html
SOURCE CAPS Medical