Centinel Spine® Commemorates 35 Years of prodisc® Clinical Leadership in an Era of Continuing Innovation
- The prodisc® platform commemorates 35 years of clinical leadership, supported by more than 540 published papers, as well as long-term evidence demonstrating sustained function with revision rates of less than 1%.
- Spine surgeon Thierry Marnay, MD, inventor or the prodisc technology, performed the first prodisc implantation in 1990 in Montpellier, France.
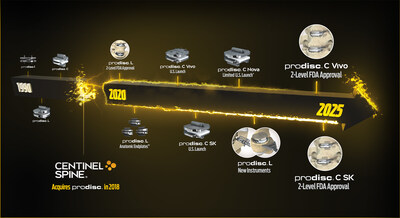
- In the last five years, Centinel Spine has ushered in a prosperous new era of total disc replacement (TDR) innovation through expanded cervical and lumbar patient indications, new anatomical implant offerings, and modernized implant and instrument systems.
- Centinel Spine's prodisc is the only TDR solution in the U.S. with FDA-approved two-level indications for both cervical and lumbar use.
WEST CHESTER, Pa., Dec. 16, 2025 /PRNewswire/ -- Centinel Spine®, LLC ("the Company"), the leading global medical device company focused exclusively on treating cervical and lumbar spinal disease with the most complete and clinically-proven total disc replacement (TDR) technology platform in the world (prodisc®), today announced the commemoration of 35 years of prodisc clinical excellence, marking the origin of modern-day total disc replacement and the evolution of the world's most studied and trusted TDR system. In the last five years, Centinel Spine has ushered in a prosperous new era of technology innovation, expanding and advancing the prodisc platform to meet the needs of today's surgeons and patients.
Since the first implantation in 1990, prodisc technology has undergone rigorous refinement, next generation engineering, expanded indications, extensive clinical trials, and multiple U.S. FDA approvals. The design and clinical durability of prodisc have been supported by more than 540 published peer-reviewed papers, with long-term evidence demonstrating sustained function and less than 1% revision rates.1 Following Centinel Spine's acquisition of the prodisc technology in 2018, the Company has accelerated its product development and regulatory efforts, culminating in a comprehensive cervical and lumbar platform that continues to advance motion preservation today.
"Innovation often starts with an obsession to solve a problem. In the case of prodisc, the challenge was finding a way to maintain natural spinal motion when treating degenerative disc disease," noted orthopedic spine surgeon Dr. Thierry Marnay, inventor of the prodisc. "For 35 years the technology has shown that preserving motion can be a reliable and lasting solution for the right patients. With more than 300,000 implantations performed, the continued success of prodisc reflects decades of clinical learning, surgeon collaboration, and disciplined engineering. This history not only validates the original principles of motion preservation and of prodisc, but also guides the next generation of advancements that will carry the technology forward for many years to come."
Building on more than three decades of prodisc clinical leadership, Centinel Spine has accelerated the evolution of the platform through its dedicated focus on total disc replacement. Since 2020, Centinel Spine's TDR achievements include:
- FDA approval of 2-level indications for the prodiscL lumbar TDR device.
- U.S. introduction of Anatomic Endplate™ options for the prodiscL system.
- Launch of the prodisc C Vivo and prodisc C SK Match-the-Disc™ cervical TDR system, providing two anatomically-distinct implant options for cervical TDR.
- Limited U.S. commercial release of the prodiscC Nova cervical TDR device, marking four unique FDA-approved prodisc devices available for cervical TDR.
- Launch of new prodisc L lumbar TDR instruments designed to streamline system ease-of-use and optimize surgical technique reproducibility.
- FDA approval of 2-level use for prodiscC Vivo and prodiscC SK cervical devices, becoming the first and only cervical TDR solution with multiple devices approved for both one-and two-level use.
Centinel Spine has also enhanced its medical education, patient access support, provider digital support assets, and ambassador & education programs to further support surgeons and patients. Together, these advancements reaffirm the Company's position as the driving force in total disc replacement as the field enters an era of accelerating adoption. This continued focus and innovation has been accompanied by strong U.S. and international growth, further expanding the global footprint of the prodisc platform.
Orthopedic surgeon Dr. Scott Blumenthal, of the Center for Disc Replacement at the Texas Back Institute in Plano, Texas, performed the first total disc replacement in the U.S. over 25 years ago. As one of the pioneers in total disc replacement, Dr. Blumenthal became the first surgeon in the U.S. to dedicate his practice exclusively to TDR and has performed over 5,000 cervical and lumbar TDR procedures since 2000.
According to Dr. Blumenthal, "Total disc replacement has been a transformative advancement and the central focus of my career for more than 25 years. I had the privilege of performing the first total disc replacement procedure in the United States and contributing to many of the studies that established the clinical foundation for disc arthroplasty. The evidence continues to affirm that this technology has far broader applicability than is currently realized. I look forward to the next quarter-century, as innovations in disc replacement evolve and utilization expands to better meet the needs of patients."
Summarizing, Centinel Spine CEO Steve Murray said, "As we enter the next era of total disc replacement, our focus remains unchanged: deliver evidence-driven innovation that meaningfully advances patient care. Over the last five years, the company has significantly strengthened and expanded the prodisc platform, modernizing the technology, broadening its clinical capability, and enhancing the tools and systems that support surgeons and patients worldwide. And this is only the beginning." He concluded, "Our innovation roadmap is robust, and our commitment to shaping the future of total disc replacement has never been stronger."
1. Based upon U.S. complaint handling units for prodisc since launch in 2006.
About Centinel Spine, LLC
Centinel Spine®, LLC is the leading global medical device company exclusively focused on addressing cervical and lumbar spinal disease with prodisc®, the most complete total disc replacement (TDR) technology platform in the world.
The Company's prodisc technology is the most studied and clinically-proven TDR system across the globe, validated by over 540 published papers and more than 300,000 implantations. Centinel Spine's prodisc is the only TDR technology with multiple motion-preserving anatomic solutions, allowing the surgeon to Match-the-Disc™ to each patient's anatomy for both cervical and lumbar total disc replacement.
For more information, please visit the company's website at www.CentinelSpine.com or contact:
Varun Gandhi
Chief Financial Officer
900 Airport Road, Suite 3B
West Chester, PA 19380
Phone: 484-887-8871
Email: v.gandhi@centinelspine.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/centinel-spine-commemorates-35-years-of-prodisc-clinical-leadership-in-an-era-of-continuing-innovation-302642674.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/centinel-spine-commemorates-35-years-of-prodisc-clinical-leadership-in-an-era-of-continuing-innovation-302642674.html
SOURCE Centinel Spine, LLC