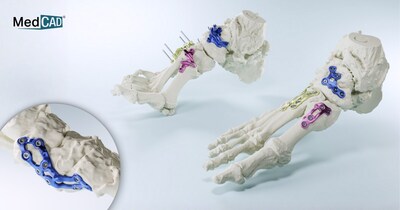
MedCAD's AccuStride™ Fixation Plates Receive 510(k) FDA Clearance to Complete Foot and Ankle System Offering
"First and Only" - AccuStride is the only FDA-cleared, truly patient-specific
3D-printed titanium implant and cutting guide system in the U.S.
DALLAS, Nov. 6, 2025 /PRNewswire/ -- Dallas-based MedCAD has been awarded 510(k) FDA clearance for its AccuStride fixation plates, part of a patient-specific solution available to surgeons as a complete foot and ankle (F&A) system. The company received 510(k) FDA clearance for its foot and ankle guides and planning system in March 2025. The AccuStride implant and surgical guide system coupled with the company's proprietary planning software will enable foot and ankle surgeons to correct multiple related pathologies in a single procedure.
"This is the first and the only FDA-cleared, truly patient-specific 3D-printed titanium implant and cutting guide system solution in the U.S.," said Nancy Hairston, CEO and president of MedCAD. "There are no other truly custom foot plates on the market, and no other truly custom comprehensive system like ours."
The AccuStride system is designed to provide truly patient-specific custom solutions for revision procedures, deformities, arthritic or diabetic conditions, and trauma-related foot and ankle injuries. Components are constructed of UV-curable acrylate polymers or titanium alloy materials and can be delivered in as few as five days after receipt of medical imaging and surgeon design approval. The system is intended for use in patients 12 years and older.
"We have great interest from leading foot and ankle orthopedic specialists who are eager to use our 'whole foot' solutions for multiple pathologies - including revisions," said Hairston. "The goal of MedCAD's portfolio of patient-matched product solutions is to provide surgeons with customizable options that are as anatomically unique as their patients."
MedCAD is also a leading manufacturer of patient-specific, custom-designed cranial and maxillofacial reconstruction implants, including virtual planning as well as surgical devices. The device is manufactured and designed to the specific individual for implantation.
"MedCAD is becoming known for putting cutting-edge planning technology into the hands of surgeons so that they can deliver life-changing outcomes for the people they heal," said Hairston. "We expect these custom 3D printed devices to reduce the frequency and duration of surgeries, and to deliver high quality, durable outcomes."
About MedCAD
MedCAD is a Dallas-based, privately held medical technology company built on an innovative approach to the design and production of patient-matched medical devices. Harnessing precise imaging, surgical experience, and advanced design and manufacturing technologies, MedCAD creates personalized, patient-matched medical devices and surgical plans for cranial defects, oral surgery, CMF trauma, and reconstructive surgical procedures. The approach is 100% patient-customized, with every device and procedure planned and manufactured in-house in cooperation with a patient's surgeon. By minimizing surgical complexity and procedure time, MedCAD technology enables superior patient outcomes throughout intervention, rehabilitation, and recovery.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/medcads-accustride-fixation-plates-receive-510k-fda-clearance-to-complete-foot-and-ankle-system-offering-302606840.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/medcads-accustride-fixation-plates-receive-510k-fda-clearance-to-complete-foot-and-ankle-system-offering-302606840.html
SOURCE MedCAD