Next-Generation AI-Driven SyncAR® Spine Receives FDA Clearance for Spine Surgery
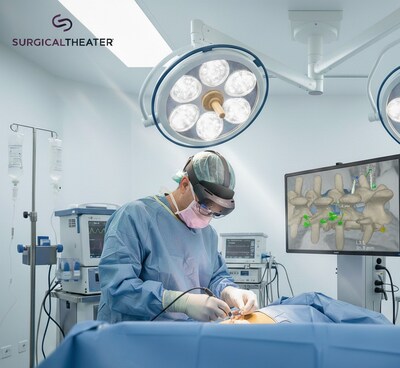
By bringing MRI and CT directly into the operating room, SyncAR Spine turns preoperative planning imaging into real-time intraoperative guidance.
CLEVELAND, Oct. 10, 2025 /PRNewswire/ -- Surgical Theater, the leader in surgical XR visualization, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for SyncAR® Spine, the next-generation release of its spine platform. This clearance expands the system's capabilities with advanced XR tools powered by AI algorithms. This brings patient MRI and CT directly into the operating room, aligning preoperative plans with intraoperative execution for greater clarity and control in spine surgery.
Building on its proven foundation in operating rooms across the country, SyncAR Spine transforms traditional imaging into a dynamic surgical roadmap, fully integrated with Medtronic StealthStation™ navigation. The platform incorporates AI-driven vertebra segmentation to streamline planning, Advanced Decompression Planning (ADP) to pre-map resections before surgery, and Segmental Fusion to keep preoperative models aligned with intraoperative CT scans even as patient positioning changes. Surgeons can track bone removal in real time with voxel-level drill tracking and, for the first time, bring MRI data, including nerves, vessels, and pathology into the OR, giving minimally invasive procedures the clarity once limited to open surgeries.
Dr. Greg Poulter, a world-renowned spine surgeon at OrthoIndy and the first to perform a SyncAR Spine case with the newly cleared next-generation platform, emphasized its impact: He emphasized its impact: "SyncAR Spine gives me the visibility of open surgery in a minimally invasive case. Having MRI data live in the OR, aligned seamlessly with navigation, is the greatest improvement in navigation since its inception nearly 2 decades ago. I can plan every detail in advance and then see that plan projected in real time during surgery, fully aligned with my workflow. That level of clarity changes how we operate."
This latest FDA clearance marks SyncAR Spine as a major evolution of the platform, validated through comprehensive testing and AI-model performance studies. It reinforces Surgical Theater's leadership in surgical XR, setting a new standard for intraoperative visualization and delivering unmatched clinical utility for spine surgery.
Surgical Theater will premiere this updated platform at two major upcoming events: the Congress of Neurological Surgeons (CNS) Annual Meeting and the North American Spine Society (NASS) Annual Meeting, where global spine leaders will have the opportunity to experience its newest capabilities firsthand.
Unlike platforms that rely on headset-only overlays or proprietary navigation, SyncAR Spine was designed as a comprehensive surgical XR platform. It integrates seamlessly with existing workflows, enhancing navigation rather than replacing it, and provides richer intraoperative visualization without disrupting surgical routines.
With more than 50,000 clinical uses within its XR platforms, Surgical Theater continues to set the standard in surgical visualization. Alon Zuckerman, President at Surgical Theater, noted, "SyncAR Spine represents the next step in that journey, bringing surgeons deeper insight, stronger alignment between plan and execution, and the unprecedented ability to carry MRI into the OR in real time. This is a transformational leap in spine surgery."
About Surgical Theater
Surgical Theater is the leader and most utilized surgical XR visualization company. Its FDA-cleared eXperiential Reality® (XR) platform harnesses AI to transform traditional 2D scans into interactive 3D models that support clinical decisions across the entire continuum of care, from the clinic to the operating room. Surgeons use XR to walk through anatomy, rehearse their plan, and align every intraoperative step with their preoperative strategy.
For media inquiries, please contact:
Tina Badat
Vice President of Marketing
Surgical Theater
714.235.7980
tbadat@surgicaltheater.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/next-generation-ai-driven-syncar-spine-receives-fda-clearance-for-spine-surgery-302580560.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/next-generation-ai-driven-syncar-spine-receives-fda-clearance-for-spine-surgery-302580560.html
SOURCE Surgical Theater