/C O R R E C T I O N -- Ampa/
In the news release, Ampa Launches Nationwide Rollout of Portable FDA-Cleared Brain Stimulation System Following $8.5M Oversubscribed Funding Round, issued Oct. 22, 2025 by Ampa over PR Newswire, we are advised by a representative of the company that the first paragraph, last sentence, should read "This milestone marks a major step in bringing advanced, non-invasive brain stimulation to patients across the country and brings Ampa's total funding to $18 million" rather than "This milestone marks a major step in bringing advanced, non-invasive brain stimulation to patients across the country" as originally issued inadvertently. The complete, corrected release follows:
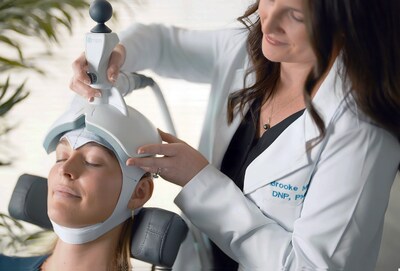
Ampa Launches Nationwide Rollout of Portable FDA-Cleared Brain Stimulation System Following $8.5M Oversubscribed Funding RoundPALO ALTO, Calif., Oct. 22, 2025 /PRNewswire/ -- Four months after emerging from stealth with FDA clearance of the Ampa One system and an oversubscribed $8.5 million round led by Nexus NeuroTech Ventures, Ampa today announced its nationwide rollout. This milestone marks a major step in bringing advanced, non-invasive brain stimulation to patients across the country and brings Ampa's total funding to $18 million.
"We've never been more depressed yet more medicated. People deserve new options," said Don Vaughn, Ph.D., neuroscientist and CEO of Ampa. "Ampa One was built to give clinicians a practical, portable, affordable tool that expands patient access to this lifesaving technology."
In addition to its FDA-cleared device, the company is developing the Ampa One Day protocol, a groundbreaking approach that condenses a traditional multi-week TMS treatment into a single day. This innovation could define a new category of rapid-acting treatments for mental health.
"Ampa represents a revolution in TMS therapy," says Tobias Marton, M.D., Ph.D., and CMO of Mindful Health Solutions. "Our clinical team has been impressed by its portability, intuitive design, and confirmed target engagement—features that redefine how and where TMS can be delivered. By enabling scalable next-generation protocols, Ampa is opening the door to broader access and better outcomes. Kudos to the Ampa team for bringing this transformative system to market."
Investors see Ampa as a company poised to reshape the mental health landscape. The company recently closed an oversubscribed Pre-A round, led by a $2 million investment from Nexus NeuroTech Ventures, a leading venture capital firm advancing treatments for brain disorders. Other institutional investors included Satori Capital, Morningside Ventures, Continuum Health Ventures, and the Zabara Foundation, alongside new and existing individual investors—physicians, TMS insiders, and mission-aligned entrepreneurs.
"Ampa is setting a new standard for what neuromodulation can look like in clinical practice," said John Propst, Ph.D., MBA, Principal at Nexus NeuroTech Ventures. "We're proud to support a company that is delivering both scientific innovation and operational scalability at the same time."
Ampa's launch into commercial deployment reflects growing momentum in the field of non-invasive brain stimulation. With hundreds of millions affected by depression, anxiety, and related conditions, the company's vision is to make remission the default, not the exception.
About Ampa
Ampa is a neurotechnology company using breakthroughs in neuroscience to create practical tools that help people recover their mental health. Its FDA-cleared Ampa One system and emerging Ampa One Day protocol advance the mission of one billion remissions from mental and neurological disorders. Learn more at www.ampahealth.com
Media Contact:
Itamar Kandel
(415) 463-3454
403271@email4pr.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/ampa-launches-nationwide-rollout-of-portable-fda-cleared-brain-stimulation-system-following-8-5m-oversubscribed-funding-round-302590882.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/ampa-launches-nationwide-rollout-of-portable-fda-cleared-brain-stimulation-system-following-8-5m-oversubscribed-funding-round-302590882.html
SOURCE Ampa