CND Life Sciences' Syn-One® Biomarker Services Used for Novel Parkinson's Disease Endpoint in Phase 2 Drug Trial
As Part of an Exploratory Outcome, ABLi Therapeutics Used CND's Syn-One Biomarker Services to Quantify and Visualize a Decrease in Phosphorylated Alpha-Synuclein in Cutaneous Nerves of Patients in a Phase 2 Trial of its Lead Drug Candidate, Risvodetinib
SCOTTSDALE, Ariz., Nov. 11, 2025 /PRNewswire/ -- CND Life Sciences, Inc. (CND), a medical technology company pioneering the development of cutaneous neurodiagnostic tests and associated biomarker solutions, today announced that its Syn-One Biomarker Services have been used to measure and quantify a reduction in the deposition of abnormal alpha-synuclein in a Phase 2 clinical trial sponsored by ABLi Therapeutics for its lead investigational Parkinson's disease (PD) treatment, risvodetinib. CND's Syn-One Biomarker Services offer biopharmaceutical companies tools to facilitate more precise patient enrollment in clinical trials, and, as an exploratory endpoint, the ability to analyze changes over time in synuclein deposition in cutaneous nerves.
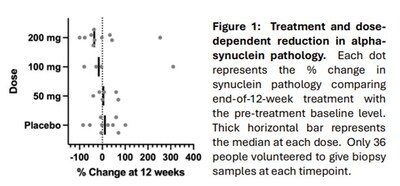
Results of ABLi's Phase 2 "201 Trial" were presented by the company's Chairman and CEO, Milton Werner, PhD, in a Clinical Breakthrough Lecture during the keynote session at the 2025 Movement Disorders Society Annual Congress on October 8, 2025, and announced in a pressrelease on October 9, 2025. In ABLi's 201 Trial, Syn-One Biomarker Services were used in an exploratory analysis examining the change in phosphorylated alpha-synuclein deposition in skin biopsy samples from baseline to 12 weeks. The analysis was conducted in a subgroup of 36 consenting subjects and showed a reduction in phosphorylated alpha-synuclein in treated subjects (see Figure 1).
"We are encouraged by the preliminary data generated using our Syn-One Biomarker Services in ABLi's 201 Trial for risvodetinib," said Christopher Gibbons, MD, chief scientific officer of CND. "While additional work needs to be done in future trials, these results support the potential role Syn-One can play in helping to quantify phosphorylated alpha-synuclein as a biomarker in clinical trials of drugs being developed to treat Parkinson's disease and other synucleinopathies."
Syn-One Biomarker Services employed in ABLi's 201 Trial included the Syn-One Research Test, which uses immunofluorescence technology to detect and visualize synuclein in cutaneous nerves, in conjunction with CND's AI-assisted digital pathology tool NerValence™, which applies deep learning methodologies to analyze cutaneous nerve structures and identify and quantify the presence of abnormal, misfolded alpha-synuclein. Developed in collaboration with Visiopharm, based in Copenhagen, Denmark, NerValence creates a visual, quantitative measurement of phosphorylated alpha-synuclein detected inside nerves, supporting the exploratory assessment of changes in synuclein deposition over time in biopharmaceutical trials.
"Syn-One Biomarker Services were used in the 201 Trial to preliminarily evaluate the effect of risvodetinib on synuclein aggregate deposition in neurons," said Dr. Werner. "ABLi remains convinced that disease modification can only be achieved by evaluating the intra-neuronal synuclein pathology that moves between neurons as a principal feature of progressive Parkinson's disease. This was the first ever measure of its kind used in a clinical trial for a Parkinson's disease drug candidate to show a possible treatment effect on intra-neuronal synuclein deposition."
In addition to offering its Syn-One Biomarker Services for use in biopharmaceutical sponsors' clinical trials, CND is continuing to study the use of the Syn-One Research Test and NerValence to quantify phosphorylated alpha-synuclein in patients with PD over time in the 18-month Syn-Q Study, which is supported by a grant from the Michael J. Fox Foundation for Parkinson's Research (MJFF).
About CND Life Sciences
CND Life Sciences supports the care of patients facing the potential diagnosis of a neurodegenerative disease. Operating a CLIA-certified and CAP-accredited laboratory in Scottsdale, Arizona, CND offers the Syn-One Test as a laboratory developed test (LDT) to help clinicians diagnose synucleinopathies that include Parkinson's disease, dementia with Lewy bodies, multiple system atrophy, and other related disorders. Syn-One uses proprietary techniques to detect phosphorylated alpha-synuclein in cutaneous nerves while also measuring other signs of peripheral nerve degeneration. Results of a prospective, multicenter NIH-sponsored study of the Syn-One Test were published in the Journal of the American Medical Association (JAMA) in 2024 demonstrating >95% sensitivity overall in patients clinically diagnosed with one of four synucleinopathies and confirmed by an expert panel.1 More than 3,000 neurologists and other clinicians have used the Syn-One Test to support their diagnostic evaluation of patients. The company also collaborates with biopharmaceutical companies on clinical trials for investigational therapies and is conducting studies on early disease detection and synuclein quantification. For more information, visit cndlifesciences.com or connect with us on LinkedIn.
About ABLi Therapeutics
ABLi Therapeutics ("ABLi") applies innovative medicinal chemistry and a deep understanding of disease biology to develop small molecule therapeutics that target the cause of diseases that arise from activation or dysfunction of the Abelson Tyrosine Kinases (c-Abl). Leveraging its expertise in drug design, ABLi utilizes clinically validated data of kinase inhibitors to design and develop novel product candidates with enhanced penetration into the brain, greater potency and target selectivity, and improved safety to treat diseases in which Abl kinase activation or dysfunction is implicated. The Company's primary focus is on developing therapeutics for the treatment of neurodegenerative diseases like Parkinson's disease and the Parkinson's-related neurodegenerative diseases Multiple System Atrophy and Dementia with Lewy Body that are all associated with Abl kinase activation or dysfunction. For more information visit www.ablitherapeutics.com or follow us on LinkedIn, Facebook or Instagram.
CND Media Contact:
Jaryd Leady
(856) 803-7855
jleady@spectrumscience.com
CND Company Contact:
Jennifer Whitney
Director, Brand Marketing
media@cndlifesciences.com
For ABLi Therapeutics
Milton H Werner PhD
Chairman & CEO
info@ablitherapeutics.com
ABLi Therapeutics Investor/Media
Mike Moyer
Managing Director – LifeSci Advisors
mmoyer@lifesciadvisors.com
References
1Gibbons CH, Levine T, Adler C, et al. Skin biopsy detection of phosphorylated α-synuclein in patients with synucleinopathies. JAMA. 2024;331(15):1298–1306. doi:10.1001/jama.2024.0792.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/cnd-life-sciences-syn-one-biomarker-services-used-for-novel-parkinsons-disease-endpoint-in-phase-2-drug-trial-302610938.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/cnd-life-sciences-syn-one-biomarker-services-used-for-novel-parkinsons-disease-endpoint-in-phase-2-drug-trial-302610938.html
SOURCE CND Life Sciences