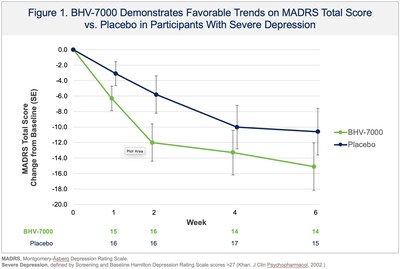
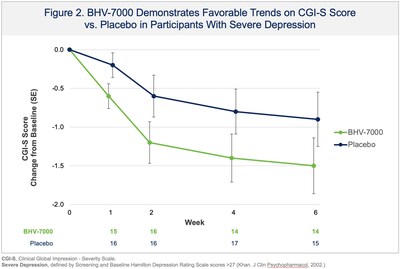
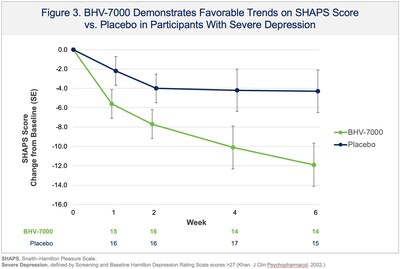
Biohaven Provides Update From Phase 2 Proof-of-Concept Study with BHV-7000 in Major Depressive Disorder
Werte in diesem Artikel
NEW HAVEN, Conn., Dec. 24, 2025 /PRNewswire/ -- Biohaven Ltd. (NYSE: BHVN) ("Biohaven"), a global clinical-stage biopharmaceutical company focused on the discovery, development, and commercialization of life-changing therapies to treat a broad range of rare and common diseases, today reported results from a Phase 2 proof-of-concept study evaluating BHV-7000 for the treatment of major depressive disorder (MDD). The study did not meet its primary endpoint, a reduction of depressive symptoms as measured by change in the Montgomery Åsberg Depression Rating Scale (MADRS) over six weeks compared with placebo. Trends favoring BHV-7000 were observed in some clinically relevant subgroups, including participants with more severe depression at screening and baseline, on primary and secondary outcome measures (see Figures 1-3). Overall, BHV-7000 was safe and well-tolerated with adverse events mostly mild and moderate in intensity and largely resolved spontaneously. The only individual adverse events occurring with an incidence above 5% were headache (10.7% and 9.9% in BHV-7000 and placebo, respectively) and nausea (4.2% and 5.6% in BHV-7000 and placebo, respectively). A low incidence of central nervous system adverse events was observed, consistent with BHV-7000's lack of GABA activity and with safety data from previously reported studies. Additional analyses are ongoing and the company plans to present the results at an upcoming scientific meeting. The company considers the depression subgroup analyses as hypothesis generating but based upon strategic prioritization of its portfolio does not plan on additional psychiatric clinical trials to keep resources focused on key priority areas of immunology, obesity and epilepsy in 2026.
Ahmed Tahseen, MD, Development Lead for Depression at Biohaven, commented, "There is an urgent need for novel therapies for depression that require the exploration of new mechanistic approaches to this common disorder. Although the results of this study do not support the efficacy of BHV-7000 in a broad population of depressed patients, we appreciate the commitment of the patients, investigators, and study teams who have advanced the field assessing new therapeutic approaches and made this important research possible."
Biohaven management will be presenting at the annual J.P. Morgan Healthcare Conference in San Francisco in January 2026 and intends to provide extensive updates across the breadth of its clinical programs, notably including:
- clinical data for two of its extracellular degrader programs from initial patient experience in the Phase 1b expansion cohorts BHV-1400 for IgAN and BHV-1300 for Graves' disease;
- expectations for the company's recently initiated Phase 2b study with taldefgrobep alfa in obesity
- oncology clinical stage assets;
- and emerging data from its ongoing clinical trial with BHV-7000 in adult focal epilepsy
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development and commercialization of life-changing treatments in key therapeutic areas, including immunology, obesity, neuroscience and oncology. Biohaven is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. Biohaven's key clinical and preclinical programs include Kv7 ion channel modulation for epilepsy and mood disorders; MoDE™ and TRAP™ extracellular protein degraders for immunological diseases; and myostatin-activin pathway targeting agents for neuromuscular and metabolic diseases, including SMA and obesity. For more information, visit www.biohaven.com.
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "potential first-in-class", "potentially", "groundbreaking" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials, including the studies of opakalim; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable US regulatory requirements; the potential commercialization of Biohaven's product candidates; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MoDE and TRAP are trademarks of Biohaven Therapeutics Ltd.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
jennifer.porcelli@biohavenpharma.com
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
mikebeyer@sambrown.com
+1 (312) 961-2502
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-provides-update-from-phase-2-proof-of-concept-study-with-bhv-7000-in-major-depressive-disorder-302649251.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/biohaven-provides-update-from-phase-2-proof-of-concept-study-with-bhv-7000-in-major-depressive-disorder-302649251.html
SOURCE Biohaven Ltd.
Ausgewählte Hebelprodukte auf Biohaven Research
Mit Knock-outs können spekulative Anleger überproportional an Kursbewegungen partizipieren. Wählen Sie einfach den gewünschten Hebel und wir zeigen Ihnen passende Open-End Produkte auf Biohaven Research
Der Hebel muss zwischen 2 und 20 liegen
| Name | Hebel | KO | Emittent |
|---|
| Name | Hebel | KO | Emittent |
|---|